22.0.0: HEAT ENERGY:
Heat is a form of energy that is transferred from one point to another due to difference in temperature between the two points. Heat energy always flow from a region of higher temperature to a region of lower temperature.
Heat energy can also be defined as a measure of the average internal energy of the particles of a substance.
UNIT OF HEAT ENEGRY:
The s.I. unit of heat energy is joule.
METHODS OF HEAT TRANSFER:
There three methods through which heat energy can be transferred from one place to another. The three methods of heat!! transfer are :
- Conduction
- Convection
- Radiation
CONDUCTION:
Conduction is a method of heat transfer in solid. The heat energy is transferred as the heated molecules vibrate about their mean position and therefore transfer heat energy from one molecules to the other until the heat energy is transferred from one point to another. It require a material medium for heat transfer.
CONDUCTORS:
Conductors are substances or materials that conduct heat energy easily. Conductors are mostly metals.
EXAMPLES OF MATERIALS THAT ARE CONDUCTORS:
Copper (ii) aluminum. (iii). Iron. ( iv). Silver
APPLICATION OF CONDUCTORS:
Conductors are used to make the followings:
- Cooking utensils
- Roofing sheets
- Cutleries
- Electrical cables
INSULATORS OR NON CONDUCTORS:
Insulators are materials that do not conduct heat energy. They do not allow heat energy to pass through them.
EXAMPLES OF INSULATORS:
- Water
- Air
- Plastics
- Wood
- Rubber
- Cotton wool
- Cork, cloth
APPLICATION OF INSULATORS:
Insulators are used to make the follow is:
Handle of cooking pots , hand gloves, foot wares, etc
THERMAL CONDUCTIVITY:
Thermal conductivity of a material or metal is the ability of the material to conduct heat energy.
EXPERIMENT TO COMPARE THERMAL CONDUCTIVITY OF DIFFERENT MATERIALS:
Aim:
To compare the thermal conductivities of different materials.
APPARATUS:
The set up diagram is as shown by the figure by your right.
PROCEDURES:
Insert equal lenght the materials into the water tank. Coat the materials with wax. Pour hot water into the water tank.
Insert equal lenght the materials into the water tank. Coat the materials with wax. Pour hot water into the water tank.
OBSERVATION:
After sometimes, it is observed that the wax on the materials melted to different lengths. The wax on copper melted most , followed by that of brass, iron and lead. The wax on the wood did not melt.
CONCLUSION:
Copper has the highest thermal conductivity followed by brass, iron and lead. Wood has the lowest thermal conductivity.
USING MOLECULAR KINETIC THEORY TO EXPLAIN CONDUCTION:
When one end of a metal is heated, the molecules of the metal gain more kinetic energy and vibrate strongly about their mean position. As they vibrate faster and faster about their mean positions, they collide strongly with the neighbouring molecules and therefore transfer heat energy to the molecules one after the other, from one end of the metal to the other end.
THERMAL CONDUCTIVITY OF LIQUID:
Water and other liquids are poor conductors of heat energy. Only mercury which is a metal in liquid form conduct heat energy.
EXPERIMENT TO DEMONSTRATE THAT WATER IS A POOR CONDUCTOR:
Copper has the highest thermal conductivity followed by brass, iron and lead. Wood has the lowest thermal conductivity.
USING MOLECULAR KINETIC THEORY TO EXPLAIN CONDUCTION:
When one end of a metal is heated, the molecules of the metal gain more kinetic energy and vibrate strongly about their mean position. As they vibrate faster and faster about their mean positions, they collide strongly with the neighbouring molecules and therefore transfer heat energy to the molecules one after the other, from one end of the metal to the other end.
THERMAL CONDUCTIVITY OF LIQUID:
Water and other liquids are poor conductors of heat energy. Only mercury which is a metal in liquid form conduct heat energy.
EXPERIMENT TO DEMONSTRATE THAT WATER IS A POOR CONDUCTOR:
AIM:
To demonstrate that water is a poor conductor
Apparatus:
Big test tube, metal gauge, water, heat source, metal wire.
SETUP OR DIAGRAM:
PROCEDURES:
Rap a piece of ice block in a metal gauze and put it into a test tube full of water. Hold the test tube in an incline position and heat the test tube at the top near the mouth of the tube.
Rap a piece of ice block in a metal gauze and put it into a test tube full of water. Hold the test tube in an incline position and heat the test tube at the top near the mouth of the tube.
OBSERVATION:
The water at the top of the tube boils. The ice at the bottom of the tube does not melt for some times.
CONCLUSION:
Water is a poor conductor that was the reason that the ice block at the bottom of the tube did not melt until after sometimes.
PRECAUTIONS:
Hold the test tube in an incline position.
Heat the water at the tope of the test tube.
Wrap the ice block with metal guaze.
PRACTICAL APPLICATION OF OF GOOD AND BAD CONDUCTORS:
Blood conductors are used in a situation whereby it is necessary to immediately conduct heat energy:
COOKING UTENSILS:
Cooking utensils such as pots and frying pans are made of good conductors such as aluminium, iron so that heat energy is quickly transferred from the fire to the contents of the pot.
FLOOR TILES FEELS COOL TO THE FEET:
When someone stand on a floor tiles, someone feels cool below the feet because the floor tiles is a good conductors of heat and conduct heat energy away from the sole of the feet. This reduced the temperature of the body and therefore make the body become cool.
PRACTICAL APPLICATION OF POOR CONDUCTORS:
USE OF RUGS ON FLOORS:
Rugs are poor conductors of heat energy. when someone stands on Rugs, someone feels warm below the feet because rugs are poor conductors of heat energy.
COOLING OF HOMES IN THE TROPICS:
Thatched roofed houses are cooler inside than galvanized iron roofed houses because thatched roof is a poor conductor of heat energy and therefore does not conduct heat energy from the outside of the house to the inside of the house unlike galvanized iron roof.
THE USE OF CLOTHES TO KEEP THE BODY WARM:
Clothes keep the body warm by trapping air between the body and the clothes. Air is a poor conductor of heat energy. Therefore the air between the body and cloth prevent the conduction of heat energy from the inside to the outside of the cloth and therefore keep the body warm.
Cooking utensils such as pots and frying pans are made of good conductors such as aluminium, iron so that heat energy is quickly transferred from the fire to the contents of the pot.
FLOOR TILES FEELS COOL TO THE FEET:
When someone stand on a floor tiles, someone feels cool below the feet because the floor tiles is a good conductors of heat and conduct heat energy away from the sole of the feet. This reduced the temperature of the body and therefore make the body become cool.
PRACTICAL APPLICATION OF POOR CONDUCTORS:
USE OF RUGS ON FLOORS:
Rugs are poor conductors of heat energy. when someone stands on Rugs, someone feels warm below the feet because rugs are poor conductors of heat energy.
COOLING OF HOMES IN THE TROPICS:
Thatched roofed houses are cooler inside than galvanized iron roofed houses because thatched roof is a poor conductor of heat energy and therefore does not conduct heat energy from the outside of the house to the inside of the house unlike galvanized iron roof.
THE USE OF CLOTHES TO KEEP THE BODY WARM:
Clothes keep the body warm by trapping air between the body and the clothes. Air is a poor conductor of heat energy. Therefore the air between the body and cloth prevent the conduction of heat energy from the inside to the outside of the cloth and therefore keep the body warm.
CONVECTION:
Convection is a method of heat transfer in liquid. The heat energy is transferred from one place to another by the movement of the heated molecules. The heated molecules move in a circular pattern. The movement of the heated molecules set up a convectional current. It require a material medium for heat transfer.
CONVECTIONAL CURRENT:
Convectional current is the movement of heated fluid molecules in a repeated circular pattern.
CONVENTIONAL CURRENT IN GASES:
Convectional currents are easily set up in gases because gases expand much more readily than liquids when their temperatures rise.
EXPERIMENT TO DEMONSTRATE CONVECTIONAL CURRENT IN GAS:
Convection is a method of heat transfer in liquid. The heat energy is transferred from one place to another by the movement of the heated molecules. The heated molecules move in a circular pattern. The movement of the heated molecules set up a convectional current. It require a material medium for heat transfer.
CONVECTIONAL CURRENT:
Convectional current is the movement of heated fluid molecules in a repeated circular pattern.
CONVENTIONAL CURRENT IN GASES:
Convectional currents are easily set up in gases because gases expand much more readily than liquids when their temperatures rise.
EXPERIMENT TO DEMONSTRATE CONVECTIONAL CURRENT IN GAS:
AIM:
To demonstrate convectional current in gas.
APPARATUS:
A wooden box with a plane glass front, two separate chimneys, candle, smoulder papaper.
The setup diagram is as shown by the figure by your right .
PROCEDURES:
Place the two chimneys at the top of the box. Lite the candle and place it under one of the chimneys. With a smoulder paper, pour smoke into the inside of the box through the second chimney.
OBSERVATION:
It is observed that the air descends down into the box. The flame of the candle warm the air, the air expands, become less dense and rises up out of the box through the other chimney. This process setup a convectional current.
CONCLUSION:
Convectional current is setup in gas.
APPLICATION OF CONVECTIN CURRENT IN COOLING DEVICES:
Place the two chimneys at the top of the box. Lite the candle and place it under one of the chimneys. With a smoulder paper, pour smoke into the inside of the box through the second chimney.
OBSERVATION:
It is observed that the air descends down into the box. The flame of the candle warm the air, the air expands, become less dense and rises up out of the box through the other chimney. This process setup a convectional current.
CONCLUSION:
Convectional current is setup in gas.
APPLICATION OF CONVECTIN CURRENT IN COOLING DEVICES:
VENTILATION:
Convectional current brings about Good ventilation in house. Air that is heated by respiration and fires, rises toward the roof and escape through ventilators near the roof. The warmed escaped air is replaced by fresh air which enters the house from outside through the windows and doors.
COOLING OF MOTOR CAR ENGINE:
Car engines are cooled to prevent overheating which will damage the engine. Connection current is used in the cooling process. The water circulates around the engine by convention current.
DOMESTIC HOT WATER SYSTEM:
Domestic hot water system works on the principle of conventional current for water circulation in the system.
EFFECT OF CONVECTIONAL CURRENT IN NATURE:
This are the natural effects that convection current produced by nature. They are:
Circulation of fresh air in a room
- Ventilation of hot room
- Land and sea breeze
SEA BREEZE:
Sea breeze is the breeze that blow from the sea to the land during the day.
DIAGRAM ILLUSTRATING SEA BREEZE:
 |
EXPLANATION OF SEA BREEZE:
During the day, the sun heat the land and the sea. The land get heated up more quickly than the sea. The air above the land become warmed, expand, become less dense and rises up to the atmosphere. The cooler and denser air above the sea surface move to the land to take the place of the warmed, expanded air that rose to the atmosphere. Also, the cooler and denser air in the atmosphere descend to the surface of the sea. As the cooler and denser air move to the land, the process repeat itself again and again. This process therefore set up a convectional current which causes air to blow from the sea to the land during the day.
LAND BREEZE:
Land breeze is the breeze that blows from the land to the sea at night.
DIAGRAM ILLUSTRATING LAND BEEZE:
The diagram that illustrates land breeze is as shown in the figure by your right.
EXPLANATION OF LAND BREEZE:
At night, the land get cooler faster than the sea. The land is cool at night while the sea is warm at night. The warmness of the sea warm air molecules above the sea surface. The air expand, become less dense and rise up to the atmosphere. The cooler and denser air above the land move to the surface of the sea to take the place of the warmed, expanded air that rose to the atmosphere. Also, the cooler and denser air in the atmosphere descend to the land. As the cooler and denser air move to the surface of the sea, the process repeat itself again and again. This process therefore set up a convectional current which causes air to blow from the land to the sea at night.
RADIATION:
Radiation is a method of heat transfer through space. The heat energy is transferred from one place to another without heating the intervening medium. Radiation does not require a material medium for heat transfer. Heat energy from a hot surface reaches us by radiation.
DETECTION OF RADIATION:
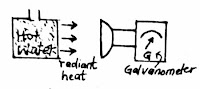
Radiometer and thermopile are used to detect radiation. A thermopile can detect and measure radiant energy. A thermopile measure and detect radiant energy by deflection of galvanometer ( a sensitive current measuring instrument) the radiant energy is converted into electrical energy by a special device. The galvanometer measure the amount of current produced from the radiant energy.
EMMISION AND ABSORPTION OF RADIANT ENERGY BY DIFFERENT SURFACES:
Different types of surfaces at the same temperature radiate or emit different amount of radiant energy. The rate or amount of radiant energy radiated by a surface depends on the nature of the surface. At a given temperature, dull black surfaces radiate heat energy most than a polished surface.
DEMONSTRATION OF RATE OF RADIATION BY DIFFERENT SURFACES:
During the day, the sun heat the land and the sea. The land get heated up more quickly than the sea. The air above the land become warmed, expand, become less dense and rises up to the atmosphere. The cooler and denser air above the sea surface move to the land to take the place of the warmed, expanded air that rose to the atmosphere. Also, the cooler and denser air in the atmosphere descend to the surface of the sea. As the cooler and denser air move to the land, the process repeat itself again and again. This process therefore set up a convectional current which causes air to blow from the sea to the land during the day.
LAND BREEZE:
Land breeze is the breeze that blows from the land to the sea at night.
DIAGRAM ILLUSTRATING LAND BEEZE:
 |
| Land breeze |
EXPLANATION OF LAND BREEZE:
At night, the land get cooler faster than the sea. The land is cool at night while the sea is warm at night. The warmness of the sea warm air molecules above the sea surface. The air expand, become less dense and rise up to the atmosphere. The cooler and denser air above the land move to the surface of the sea to take the place of the warmed, expanded air that rose to the atmosphere. Also, the cooler and denser air in the atmosphere descend to the land. As the cooler and denser air move to the surface of the sea, the process repeat itself again and again. This process therefore set up a convectional current which causes air to blow from the land to the sea at night.
RADIATION:
Radiation is a method of heat transfer through space. The heat energy is transferred from one place to another without heating the intervening medium. Radiation does not require a material medium for heat transfer. Heat energy from a hot surface reaches us by radiation.
DETECTION OF RADIATION:
Radiometer and thermopile are used to detect radiation. A thermopile can detect and measure radiant energy. A thermopile measure and detect radiant energy by deflection of galvanometer ( a sensitive current measuring instrument) the radiant energy is converted into electrical energy by a special device. The galvanometer measure the amount of current produced from the radiant energy.
EMMISION AND ABSORPTION OF RADIANT ENERGY BY DIFFERENT SURFACES:
Different types of surfaces at the same temperature radiate or emit different amount of radiant energy. The rate or amount of radiant energy radiated by a surface depends on the nature of the surface. At a given temperature, dull black surfaces radiate heat energy most than a polished surface.
DEMONSTRATION OF RATE OF RADIATION BY DIFFERENT SURFACES:
AIM:
To demonstrate rate of radiation of surfaces.
APPARATUS:
Leslie cube, thermopile, different colour surfaces, hot water
SETUP / DIAGRAM:
The diagram that illustrates the Leslie cube and thermopile is as shown by the figure by your right.
PROCEDURES:
Paint the faces of the cube with dull black, grey colour, bright colour. Pour hot water into the cube. Turn each of the surfaces to face the thermopile one after the other.
 |
| Leslie cube and thermopile |
PROCEDURES:
Paint the faces of the cube with dull black, grey colour, bright colour. Pour hot water into the cube. Turn each of the surfaces to face the thermopile one after the other.
OBSERVATION:
It is observed that the dull black surface produced the most deflection followed by the grey surface and bright surface.
CONCLUSION:
Since the dull black surface produced the most deflection followed by the grey surface and bright surface, the dull black surface produced the most radiation, followed by the grey surface. bright surface produced the least radiation.
EXPERIMENT TO SHOW THAT BLACK SURFACE IS ALSO A BETTER ABSORBER OF HEAT THAN A POLISHED SURFACES:
AIM:
To show that a black surface is a better absorber of heat than a polished surface.
APPARATUS:
A dull black surface, a polished surface, candle, wax, two pieces of corks.
SETUP / DIAGRAM:
 |
| Heat absorption of different surfaces |
PROCEDURES:
Coat the two surfaces with wax and fix a cork on each surfaces. Place a lit candle at equal distance between the two surfaces.
OBSERVATION:
It is observed that after sometimes, the wax on the dull black surface melted and the cork fell off the surface. While the corkmon the polished surface remain in position.
CONCLUSION:
Since the wax on the dull black surface melted earlier than that on the polished surface, dull black surface absorbs heat energy morevthan polished surface.
PRECAUTIONS:
Apply the amount of wax on the surfaces. Place the lit candle at the centre between the two surfaces. Use the same size of cork.
THE THERMO FLASK OR VACUUM FLASK:
Thermo flask is used to keep the temperature of its contents constant over a period of time.
CONSTRUCTION OF THERMO FLASK:
Thermo flask is made of a silvered surface and doubled wall vacuum placed on a cork. The composition is placed in a housing to give it a perfect design.
 DIAGRAM OF THERMO FLASK:
DIAGRAM OF THERMO FLASK:The figure by your right show the construction of thermo flask and the different parts of the flask.
PARTS OF THERMO FLASK AND THEIR FUNCTIONS:
- The doubled wall:
- Silvered surface:
- Cork:
EXERCISES:
- Differentiate the three methods of heat energy transfer.
- What is a thermo flask? Explain the features of a thermo flask and their functions.
- What is heat energy? State its unit.
- What are the applications of conductors.
- List and explain the natural effects of convection current.
- How can you show that water is a poor conductor?
- What is conductivity? Explain an experiment to compare the conductivities of different metals.
- Differentiate between a radiometer and a thermopile.
- What are the composition of a thermopile? How can it be used to measure the radiant energy of surfaces?



Splendid
ReplyDeleteEnter your comment...nicee
ReplyDeletegreat job
I love this so helpful
ReplyDeleteIt’s INCREDIBLY hard to visualize data with energy flow characteristics. It turns out there’s a chart that’s strategically positioned to visualize data with flow characteristics. Besides, this chart is amazingly easy to read and interpret. How to create https://ppcexpo.com/blog/energy-flow-diagram
ReplyDeleteGood job, keep it up please.
ReplyDeletenice ,thanks for the helpful answer
ReplyDeleteThnx
ReplyDelete